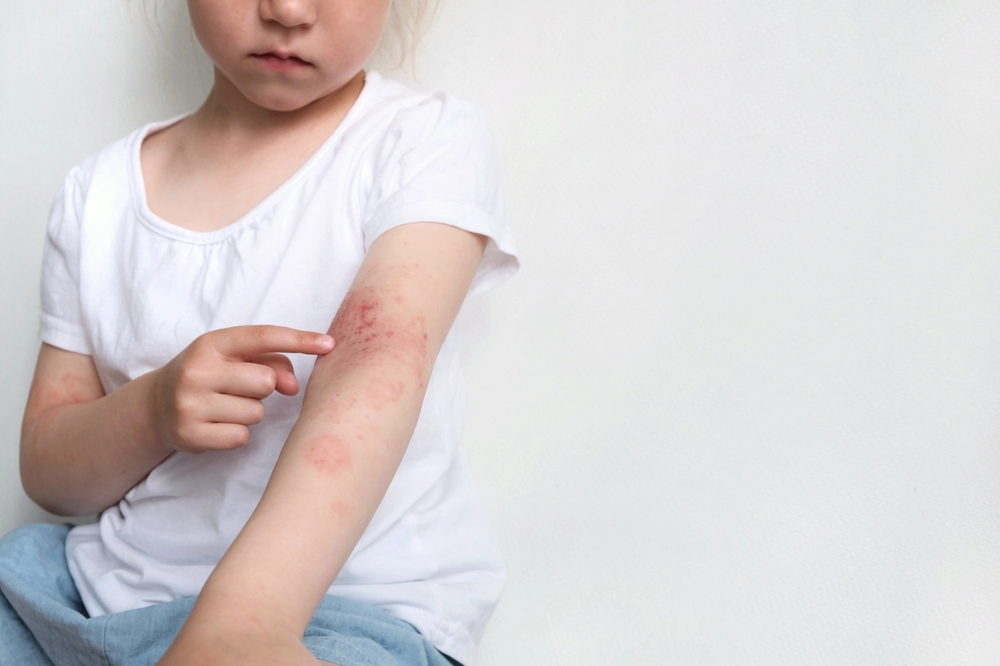
Dermavant Sciences has submitted a supplemental new drug application to the FDA for VTMA (tapinarof) cream 1%, a treatment for atopic dermatitis (AD) in patients aged 2 years and older.
Dermavant Seeks FDA Approval for Tapinarof Cream in Treating Young Patients with Atopic Dermatitis
Kate Bondaruk2024-03-28T14:50:54+00:00March 28th, 2024|Dermatology|Comments Off on Dermavant Seeks FDA Approval for Tapinarof Cream in Treating Young Patients with Atopic Dermatitis
Related Posts
-
Itch in Psoriasis and Atopic Dermatitis: Pathways and Impact on Quality of Life
September 16th, 2024